Mission 1
We're establishing health security by securing vaccine and treatment sovereignty through timely R&D support.
This allows us to respond to government-wide and societal threats like infectious diseases and biochemical terrorism.
-
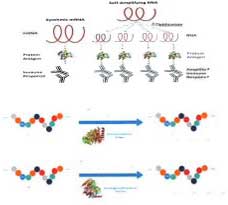
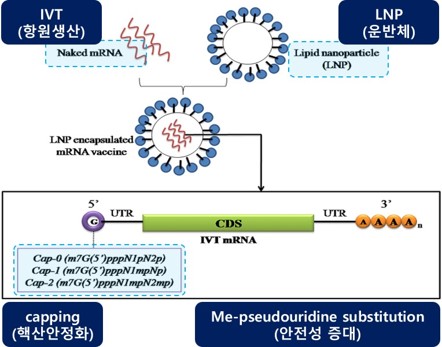
- STOREx
Stockpile Technology to Omit Repeated Entityfor Vx
- Development of Vaccine Technology for Ultra-Long-Term Stockpile
(Development of Ultra-Long-Term Vaccine Stockpiling Technologies)
- STOREx
-
- DeCAFx
DeCentralizing & Accelerating Facility for Vx
- Development of a modular production system capable of ultra-rapid production of vaccines for pandemic response
(Development of a decentralized vaccine production system)

- DeCAFx
-
- PROCUREx
Pandemic Readiness for Outbreak Control & Urgent REscue with Tx
- Development of therapeutics to suppress severe cases for responding to infectious disease pandemics

- PROCUREx