Government Investment in Biotechnology (2002~2011)
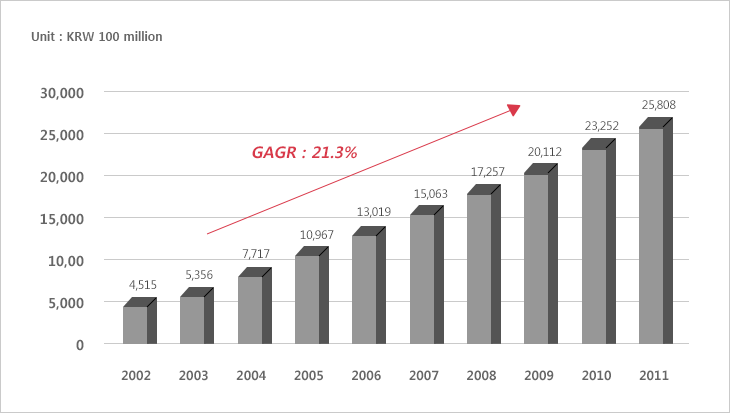
Source : Biotech Policy Research Canter 2013
In biotechnology R&D, the Korean government's investment began to increase conspicuously from the 1990s and the investment exceeded 2 trillion won in 2009 and reached 2.58 trillion won in 2011.
New Drug R&D Pipelines of Major Korean Pharmaceutical Companies by Therapeutic Category
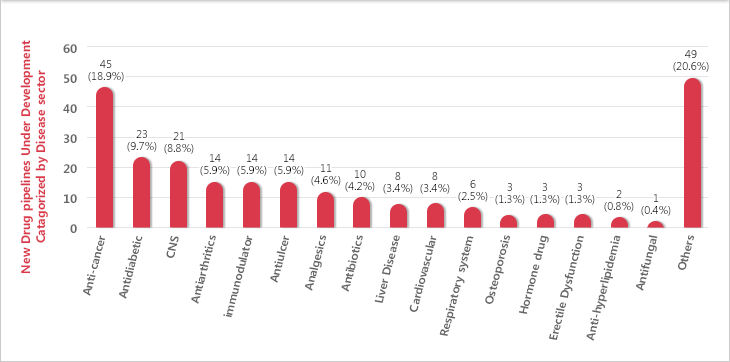
Source : kDRA Suvey over 37 Leading pharmaceutical companies in 2012
New Drug R&D Pipelines of Major Korean Pharmaceutical Companies by R&D Stage
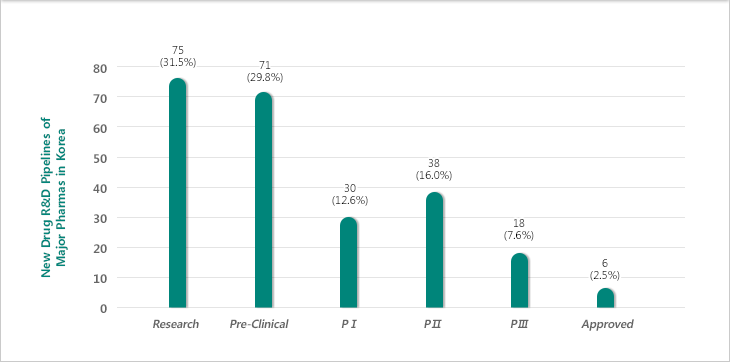
Source : kDRA Suvey over 37 Leading pharmaceutical companies in 2012
- New Drug R&D Pipelines of major korean pharmaceutical companies have been mainly undertaken in fields of cancers, diabetes and central nervous system(CNS).
- Most of the pipelines by R&D stage are in research and preclinical stage.
Biotechnology & NSC theses Performance
Publications from Korea in biotechnology by year
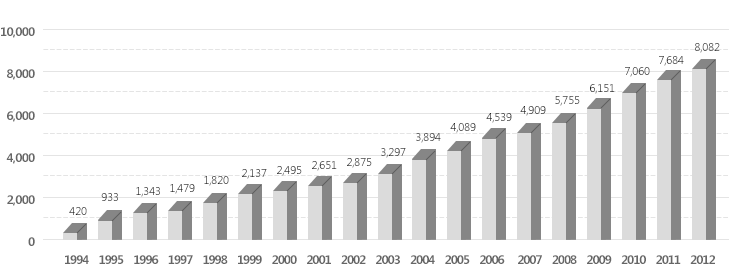
Analysis : Biotech Policy Research Center, 2013
Publication capacity of papers from Korea in biotechnology is rapidly growing every year, and quality of papers is also increased upon publishing in highly prestigious international journals.
Domestic publications in NSC (2001~2012)
| Year | ‘01 | ‘02 | ‘03 | ‘04 | ‘05 | ‘06 | ‘07 | ‘08 | ‘09 | ‘10 | ‘11 | ‘12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NSC publications total | 14 | 19 | 13 | 18 | 29 | 28 | 26 | 28 | 38 | 47 | 44 | 43 |
| BT-related NSC publications total |
11 | 11 | 11 | 10 | 23 | 18 | 19 | 15 | 21 | 28 | 25 | 22 |
| BT ratio (%) | 78.6 | 57.9 | 84.6 | 55.6 | 79.3 | 64.3 | 73.1 | 53.5 | 55.2 | 59.5 | 56.8 | 51.2 |
papers from Korea, published in world-renowned scientific journals such as Nature, Science and Cell(NSC), have been increasing in number, more than 60% of which refer to biotechnology. During last five years (2008~2012), 222 papers have been published on NSC (18th in the world).
Clinical Trials
Clinical Trials Approved by KFDA

Source : KFDA, 2013
- The Korea Food & Drug Administration (KFDA) approved 303 cases of multinational clinical trials and 367 cases of domestic clinical trials.
- Phase I clinical trials conducted in Korea have considerably increased from 19 cases in 2011 to 32 cases in 2012.
