Approval Process
home Korea Pharmaceutical Industry Information Drug Regulations Approval Process
Overview of Pharmaceutical Approval Process
Approval is granted to pharmaceuticals for which the safety, efficacy, and quality are verified (including pharmaceutical ingredients) through technological review and inspection for their manufacturing and distribution. (「Pharmaceutical Affairs Act」 Article 31)
- Classification of pharmaceuticals: Drug products; Pharmaceutical ingredients
- Classification of drug products: New drug; Pharmaceutical required for data submission; and Generic drug
- 1 The term 'New Drug' refers to a new pharmaceutical material of which the chemical structure or original composition is totally different from pharmaceuticals previously approved in Korea, or a multiple preparation containing a new material as an active drug substance, which is designated by the Minister of Food and Drug Safety. (「Pharmaceutical Affairs Act」Article 2 Subparagraph 8)
- 2 The term 'Pharmaceutical Required for Data Submission' refers not to a new drug but a pharmaceutical required for the evaluation of safety and efficacy. [Regulation on Pharmaceuticals Approval, Notification and Review (MFDS Notification) Article 2 Subparagraph 8], e.g., pharmaceuticals with new effectiveness, new composition or increase/decrease in the strength of active drug substance, and pharmaceuticals with new route of administration, etc.
- 3 The term 'Generic Drug' refers to a pharmaceutical that is equivalent to a new drug (reference drug) in terms of active ingredient, dosage form, and strength.
Data Requirements for Approval
- New Drug ([Regulation on Safety of Pharmaceuticals (Ordinance of the Prime Minister) Article 9]
- (Review by the Drug Evaluation Department) Safety and efficacy data, specifications and test methods, Drug Master Files (DMF),certificate of manufacturing and marketing (Imported Pharmaceutical), data such as the name and address of the manufacturers of active pharmaceutical ingredients
- (Review by Other Departments) Data based on conducting the evaluation of Good Manufacturing Practice (GMP)
Figure 1. Dossier for Evaluation of Safety and Efficacy
- Origin or background leading up to the discovery and development
- Stability test data
- Drug substances
- Drug product long-term accelerated, stressed
- Pharmacologic effects
- Data on efficacy studies
- Data based on safety or general pharmacology studies
- ADME
- Other pharmacologic effects
- Uses in other countries
- Structure: Physical, chemical and biological nature
- Drug substance
- Drug product
- Toxicity
- Single dose toxicity
- Repeated dose toxicity
- Genetic toxicity
- Carcinogenicity
- Reproductive and developmental toxicity
- Other antigenicity, immunogenicity, local toxicity dependency, etc.
- Clinical data
- Clinical data package
- Bridging data
- Comparison with domestic copies & Special features of the drug concerned
Figure 2. Dossier with Evaluation of Data on Specifications and Test Method
- A Data on drug substance
- Data on structure identification
- Data on physical and chemical properties
- Data on manufacturing methods
- Data on specifications and test methods are stated
- Supportive data on specifications and test methods
- Data on test results
- Data on reference standards, reagents, and test solutions
- Data on containers and packaging materials
- B Data on drug product
- Data on composition
- Data on manufacturing methods
- Data on specifications and test methods are stated
- Supportive data on specifications and test methods
- Data on test results
- Data on reference standards, reagents, and test solutions
- Data on containers and packaging materials
Pharmaceutical requiring data submission: Selectively submit data required for the evaluation of safety and efficacy among submission data for new drugs.
Generic drugs: Submit bioequivalence test data and quality data instead of safety and efficacy data such as toxicity, pharmacology, clinical trials. 「Regulation on Safety of Pharmaceuticals」(Ordinance of the Prime Minister) Article 4 (1) 3* * Generic drugs are reviewed by the Drug Evaluation Department and approved by regional FDAs
Work Flow for Approval of Pharmaceuticals
New drug, pharmaceutical requiring data submission
- Submission
- Drug Review Management Division
Evaluate user fee based on a related regulation
- Drug Review Management Division
- Pre-review
- Drug Review Management Division
- Composition of preliminary report
- Assign a product manager (PM) *
- Identify the applicable regulations
- Check history of civil petition
- Check whether requested for product briefing *
- Check Incrementally Modified Drug (IMD) etc.
- Drug Review Management Division
- Review
- Pharmaceutical Standardization Division
- Cardiovascular & Neurology Products Division
- Oncology & Antimicrobial Products Division
- Gastroenterology & Metabolism Products Division
- Bioequivalence Evaluation Division
- Approval
- Drug Review Management Division
- Designate as new drug, IMD, drug required for re-examination
- Manage review results such as efficacy and effectiveness
- Limit approval conditions
- Delist the specifications
- Open approval information
- Drug Review Management Division
- Issue
- Customer Support Office
Issuing certificate of approval results
- Customer Support Office
- Inspection of manufacturing sites and management practices
Overseas manufacturing site (Pharmaceutical Quality Division); Domestic manufacturing sites (at Medical Product Safety Division of regional FDAs)
- Consult with Central Pharmaceutical Affairs Advisory Committee (CPAC), if necessary during the overall process of drug review and approval
- Product Manager (PM) serves as a general manager to oversee the entire process from submission, pre-review, supplementation, approval, and revision for each product.
- Pre-review system: The PM examines submission data and the adequacy of data requirements
- Product Briefing
- (Priority) New drug, IMD and any other information requested by civil petitioners
- (Participants) Civil petitioners, Review Division, Pharmaceutical Policy Division: Improve the efficiency and predictability of the review and approval process for the relevant products by enhancing mutual understanding between the reviewer and petitioner
- Drug Approval Update
- Weekly withdrawals, monthly approvals, approval reports (NDA), results of safety and efficacy evaluations (pharmaceuticals requiring data submission)
Generic Drugs
Required Application Documents: Bioequivalence study, GMP documents and CMC (Chemistry, Manufacturing, Controls) data
Figure 1. The Process of Generic Drugs Approval < New drug/ pharmaceutical that requires data submission
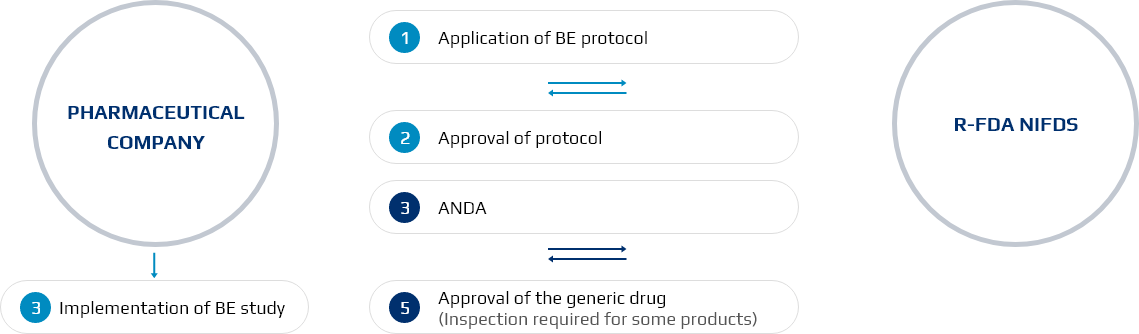

- RFDA approves generic drugs via drug approval evaluation
- Consult with Central Pharmaceutical Affairs Advisory Committee (CPAC), if necessary during the whole process of drug review and approval.