KHIDI supports initiatives to protect the rights and interests of foreign patients, and promotes the convenience of using medical care in Korea so that patients can receive safe and high-quality medical and healthcare services.
Through intergovernmental cooperation, KHIDI enhances the international competitiveness of the nation's healthcare industry and contributes high added value to the industry through job creation.
Key Results of Global Healthcare Promotion
- Overseas expansion of Korean medical institutions (Year 2016-September 2020)
20countries / 89cases
- Certified Innovative Pharmaceutical Companies: Total number of companies and amount of government funding (Including R&D and program support, tax benefits, and price incentives/Year2018)
7.09 million persons
- Foreign medical practitioners invited for training (Years 2007-September 2020)
32 countries / 864 persons
Worldwide recognition of Korean medical services
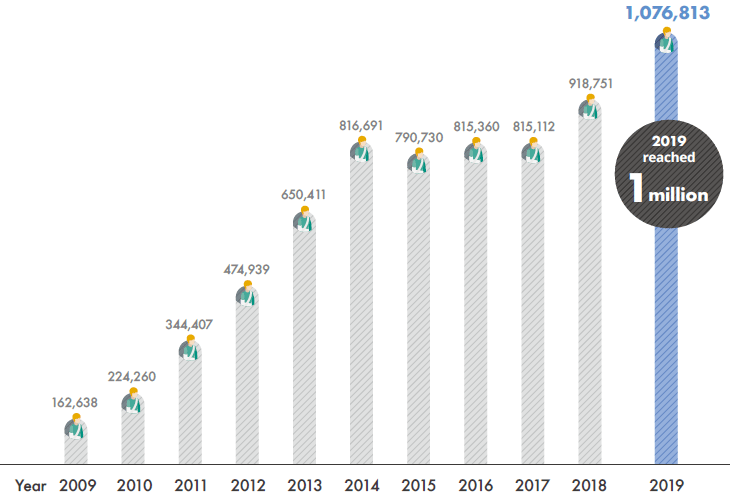
- No. of foreign patients
(Year 2009-2019)7.09 million persons
- No. of countries where foreign
patients came from (Year 2009-2019)199countries
The total number of medical treatments for foreign patients per year
Since 2009 when Korean medical institutions were officially allowed to accept foreign patients, the accumulated number of foreign patients who visited Korea amounted to 7.09 million, while the annual figures reached 1 million in 2019.
* KHIDI analyzes the statistics of foreign patients on a yearly basis.
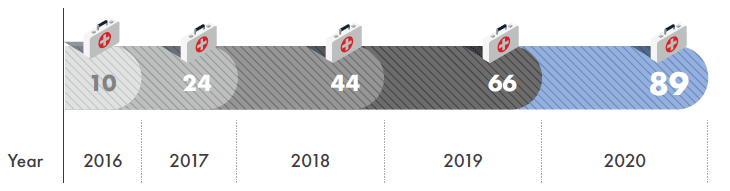
Overseas expansion of Korean medical institutions (since 2016 when reporting system was established)
- Accumulated no. of cases (Year 2016 - September 2020)
20countries / 89cases
- Overseas Expansion of Korean Medical Institutions (Unit : No. of cases)
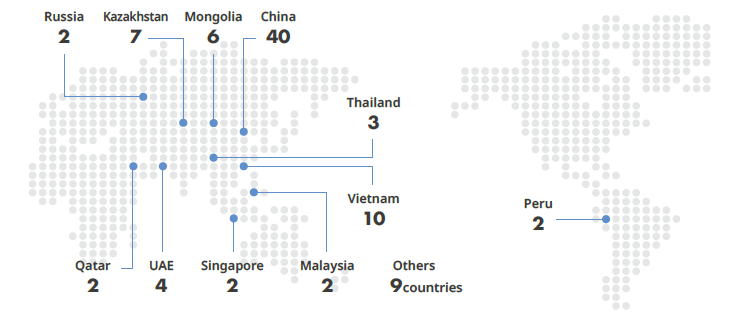
- Overseas Expansion of Korean medical institutions by countries (Unit : no. of cases)
MEDICAL KOREA
-
 Announcement of 'MEDICAL KOREA' brand
Announcement of 'MEDICAL KOREA' brand -
 Signed Renewal Contract for consignment management of Seoul National University Hospital - UAE Sheikh Khalifa Specialist Hospital
Signed Renewal Contract for consignment management of Seoul National University Hospital - UAE Sheikh Khalifa Specialist Hospital
Satisfaction level of foreign patients
- Satisfaction level of foreign patients who experienced Korean medicine (2018 : 90.5 points ‣ 2019 : 91 points)
- Foreign patients showed a high level of satisfaction in a variety of areas such as "service", "hospital convenience", "accessibility", "communication and respect towards patients", "hospital accommodation", "provision of information and training", and "medical expenses" etc.
- Awarded ‘Health and Medical Tourism : Destination of Year’ for two consecutive years at the ‘IMTJ Medical Travel Awards’ (2018, 2019)
- Ranked 1st place for two consecutive years in terms of awareness and preference amongst medical tourism destinations in the ‘Foreign Awareness Survey on Korea Medical Wellness’(Korea Tourism Organization), which started in 2018
Creation of a Convenient and Safe Environment for Korean Medical Services
-
 Medical consultant service
Medical consultant service
(photo provided by SNU Gangnam center) -
 Foreign patients getting consultations at the MEDICAL KOREA Information Center
Foreign patients getting consultations at the MEDICAL KOREA Information Center
- Provision of online/offline information on medical services, tourism, and wellness to foster convenient use of Korean medical services
- Various information on topics such as medical institutions, medical technology, and wellness is provided on www.visitmedicalkorea.com in 5 languages (English, Chinese, Japanese, Russian, Arabic)
- Operation of MEDICAL KOREA Information Center (Incheon International Airport and Myeongdong, Seoul)
* Multilingual consultation and information provided on medical service use, beauty·cosmetic tax returns, and connection to medical translators
- Provision of policy support and implementation of medical institutions evaluation and certification program to protect foreign patient's rights and interest, to create a safe medical environment, and to provide high-quality medical services.
- We provide information on reporting procedures for illegal brokers and consultation/information in case of medical disputes between foreign patients and medical institutions. If needed, we also support connecting the Korea Medical Dispute Mediation with the Arbitration Agency or the Korea Consumer Agency.
- The Korean government operates an accreditation program (KAHF) that evaluates and designates selected medical institutions with state-of-art international medical service standards.
Global CSR and creation of shared values among Korean medical institutions
-
 Korean Medical Charity Program Event
Korean Medical Charity Program Event -
 Celebration for Returning Home of the Medical Charity Program Patient
Celebration for Returning Home of the Medical Charity Program Patient
We disseminates a value of international contributions by focusing on core competencies and resources of the public and private medical institutions to realize the core value of medical care through co-prosperity beyond competition in the global healthcare market.
Complimentary Medical Treatment via Medical Charity Program
- Medical Charity Program
Implementation of the ‘Korean Medical Charity Program,’ which invites foreign patients with a lack of access to healthcare services and provides the necessary treatment they are unable to receive elsewhere - 404 patients from 26 countries received complementary medical treatment via the Korea Medical Charity Program from 2011 to 2019.
-
 Medical Charity Program(Peru)
Medical Charity Program(Peru) -
 Medical Charity Program(Mongolia)
Medical Charity Program(Mongolia)
-
- Medical KOREA ACADEMY
‘MEDICAL KOREA ACADEMY’ provides opportunities for outstanding foreign medical practitioners to get professional training from Korean medical institutions
- Observation Course (Medical Korea Academy-Global, Korea-Mongolia, Korea-Russia) : completed by 703 persons from 32 countries
- Clinical Training Course (Middle Eastern Physician & Dentist) : completed by 161 persons from 4 countries (Saudi Arabia, Kuwait, Bahrain, Oman)
- Initiating the Online Training Program (MKA e-class) to prepare for the post-COVID era
No. of Trainees who Completed Medical Programs
- No. of trainees who completed the Medical Observation Program
(Years 2007- September 2020)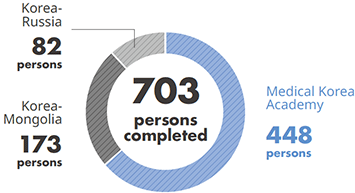
- No. of trainees who completed the Medical Clinical Program
(Years 2015- September 2020)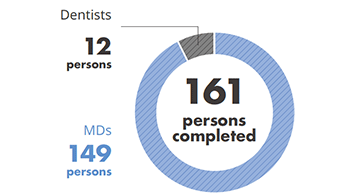
- MEDICAL KOREA, a global healthcare event, held annually since 2010 to exchange the latest information on healthcare sector and to provide a place for networking.
Hosting of conferences, B2B(business meetings), Korean hospital experience event, exhibition & PR hall

